EST. 2014
Publications
(images are links, corresponding authors are bold)
[45] Oh, S. M.; Lee, V. S.; Drayer, W. F.; Win, M. S.; Jones, L. F.; Leo, C. L.; Kennemur, J. G.; Frischknecht, A. L.; Winey, K. I. Effect of Sulfonation Level on the Percolated Morphology and Proton Conductivity of Hydrated Fluorine-Free Copolymers: Experiments and Simulations. JACS Au, 2025, 5, 2641–2653.
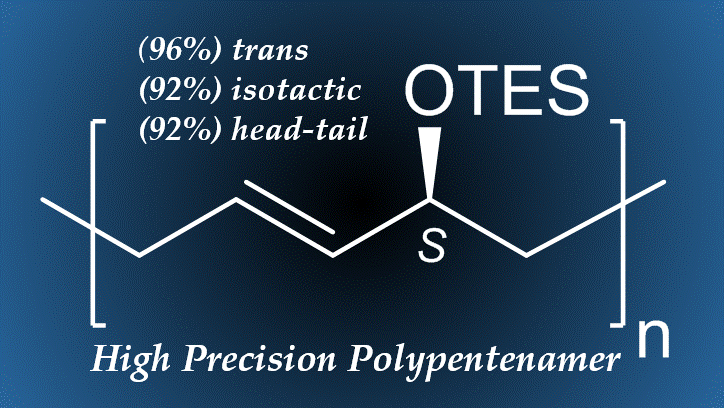
[34] Coia, B. M.; Werner, S.; Kennemur, J. G. Conformational Bias in Density Functional Theory Ring Strain Energy Calculations of Cyclopentene Derivatives: Towards Predictive Design of Chemically Recyclable Elastomers. J. Polym. Sci., 2022, 60, 3391. (Invited Submission - Treating the Plastics Problem: From Renewable Feedstocks and Degradability to Recycling)
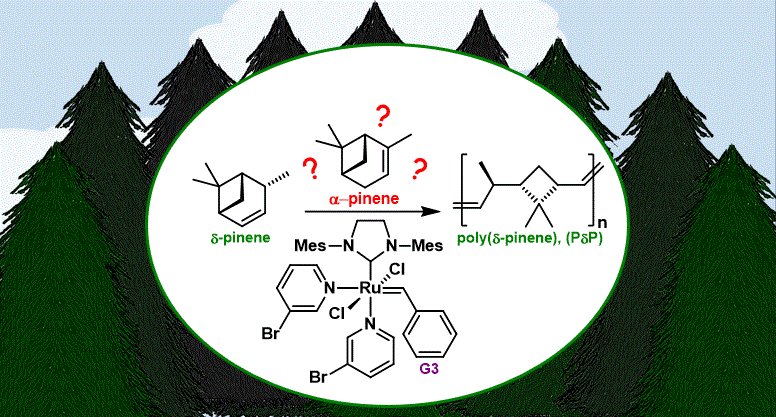
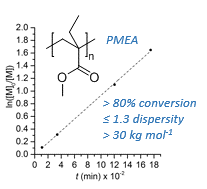
[30] Yarolimek, M. R.; Coia, B. M; Bookbinder, H. R.; Kennemur J. G. Investigating the effect of α-pinene on the ROMP of δ-pinene. Polym. Chem.. 2021, 12. 5048–5058
(Themed Collection: Sustainable Polymers 2021)
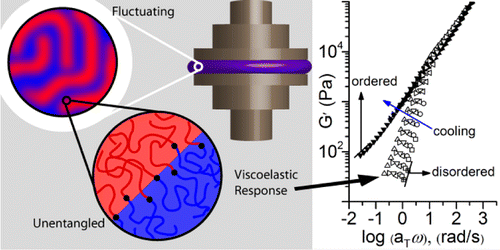
[26] Paren, B. A.; Thurston, B. A.; Neary, W. J.; Kendrick, A.; Kennemur J. G.; Stevens, M. J.; Frischknecht, A. L.; Winey, K. I.* Percolated Ionic Aggregate Morphologies and Decoupled Ion Transport in Precise Sulfonated Polymers Synthesized by Ring-Opening Metathesis Polymerization effects. Macromolecules, 2020, 53, 20, 8960-8973
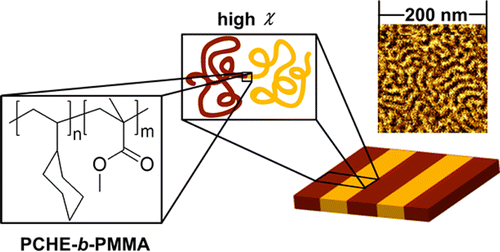
[11] Misichronis, K.; Chen, J.; Imel, A.; Kumar, R.; Thostenson, J.; Hong, K.; Dadmun, M.; Sumpter, B. G.; Kennemur, J. G.; Hadjichristidis, N.; Mays, J. W.; Avgeropoulos, A. Investigation on the Phase Diagram and Interaction Parameter of Poly(styrene--1,3-cyclohexadiene) Diblock Copolymers. Macromolecules, 2017, 50, 2354-2363.
Prior to Independent Career
Patents
6. Kennemur, J. G.; Yarolimek, M. A. Polymers from Biomass. US Patent 11,897,987 B2. Feb. 13, 2024.
5. Kennemur, J. G.; Fultz B. A. Block Copolymers, Membranes and Methods US Patent 11,459,420 B2. Oct. 4, 2022.
4. Kennemur, J. G.; Cyclopentene Monomers and Methods of Polymerization. U.S. Patent 11,136,426. Oct. 15, 2021.
3. Kennemur, J. G.; Neary W. J. Polystyrene Sulfonate Analogs and Methods. U.S. Patent 10,640,587. May 5, 2020.
2. Hustad, P. H.; Bates, F. S.; Hillmyer, M. A.; Kennemur, J. G. Poly(cyclohexylethylene)-Polyacrylate Block Copolymers, Methods of Manufacture Thereof and Articles Comprising the Same. U.S. Patent (2014) 20140360975A1.
1. Hustad, P. H.; Trefonas, P. F.; Bates, F. S.; Hillmyer, M. A.; Kennemur, J. G. 2011. Polystyrene-Polyacrylate Block Copolymers, Methods of Manufacture Thereof and Articles Comprising the Same. U.S. Patent (2013) 9,127,113.